

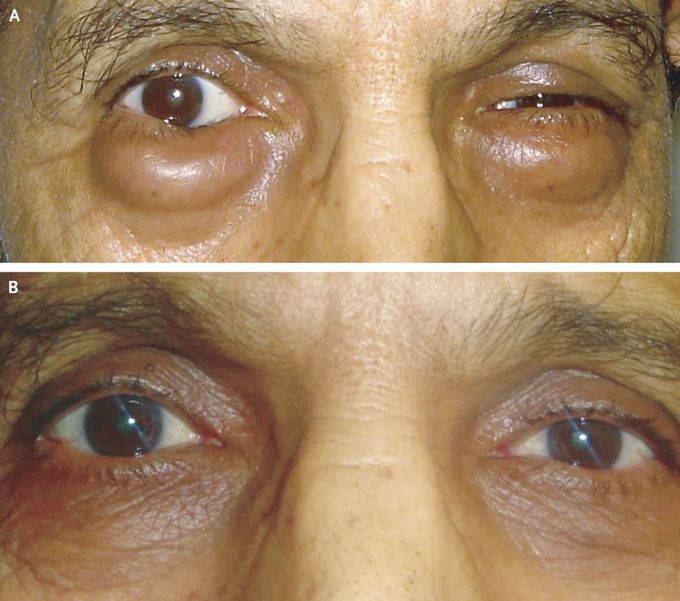
Bilateral Lower Palpebral MALT Lymphoma
A 65-year-old man presented with a 2-month history of painless swelling of the lower eyelids bilaterally. He had had a permanent pacemaker inserted 4 years previously for the treatment of third-degree heart block. On physical examination, bilateral palpebral edema and palpable masses were identified (Panel A). Computed tomography (CT) of the head showed enlargement of both lower eyelids with no associated cervical lymphadenopathy. Excision biopsy of the palpebral tumor revealed mucosa-associated lymphoid tissue (MALT) lymphoma. Comprehensive physical examination revealed no other lesions. Localized MALT lymphoma of the lower eyelids was diagnosed. The patient was treated with radiation therapy, delivered in five daily fractions of 3.2 Gy, and had a complete response after the completion of radiation therapy (Panel B). At 18 months of follow-up, ophthalmologic examination and CT revealed no relapse of lymphoma at a local or distant site.
Treatment Use of Investigational Drugs Investigational products are sometimes used for treatment of serious or life-threatening conditions either for a single subject or for a group of subjects. The procedures that have evolved for an investigational new drug (IND) used for these purposes reflect the recognition by the Food and Drug Administration (FDA) that, when no satisfactory alternative treatment exists, subjects are generally willing to accept greater risks from test articles that may treat life-threatening and debilitating illnesses. The following mechanisms expand access to promising therapeutic agents without compromising the protection afforded to human subjects or the thoroughness and scientific integrity of product development and marketing approval. OPEN LABEL PROTOCOL OR OPEN PROTOCOL IND These are usually uncontrolled studies, carried out to obtain additional safety data (Phase 3 studies). They are typically used when the controlled trial has ended and treatment is continued so that the subjects and the controls may continue to receive the benefits of the investigational drug until marketing approval is obtained. These studies require prospective Institutional Review Board (IRB) review and informed consent. TREATMENT IND The treatment IND [21 CFR 312.34 and 312.35] is a mechanism for providing eligible subjects with investigational drugs for the treatment of serious and life-threatening illnesses for which there are no satisfactory alternative treatments. A treatment IND may be granted after sufficient data have been collected to show that the drug "may be effective" and does not have unreasonable risks. Because data related to safety and side effects are collected, treatment INDs also serve to expand the body of knowledge about the drug. There are four requirements that must be met before a treatment IND can be issued: 1) the drug is intended to treat a serious or immediately life-threatening disease; 2) there is no satisfactory alternative treatment available; 3) the drug is already under investigation, or trials have been completed; and 4) the trial sponsor is actively pursuing marketing approval. Treatment IND studies require prospective IRB review and informed consent. A sponsor may apply for a waiver of local IRB review under a treatment IND if it can be shown to be in the best interest of the subjects, and if a satisfactory alternate mechanism for assuring the protection of human subjects is available, e.g., review by a central IRB. Such a waiver does not apply to the informed consent requirement. An IRB may still opt to review a study even if FDA has granted a waiver. Treatment INDs are discussed under the general heading of expanded access to investigational drugs. On August 13, 2009, FDA issued in the Federal Register 21 CFR Part 312 and 316, Charging for Investigational Drugs Under an Investigational New Drug Application; Expanded Access to Investigational Drugs for Treatment Use; Final Rules. These rules include clinical studies conducted under an IND as well as treatment protocols and treatment INDs. GROUP C TREATMENT IND The "Group C" treatment IND was established by agreement between FDA and the National Cancer Institute (NCI). The Group C program is a means for the distribution of investigational agents to oncologists for the treatment of cancer under protocols outside the controlled clinical trial. Group C drugs are generally Phase 3 study drugs that have shown evidence of relative and reproducible efficacy in a specific tumor type. They can generally be administered by properly trained physicians without the need for specialized supportive care facilities. Group C drugs are distributed only by the National Institutes of Health under NCI protocols. Although treatment is the primary objective and patients treated under Group C guidelines are not part of a clinical trial, safety and effectiveness data are collected. Because administration of Group C drugs is not done with research intent, FDA has generally granted a waiver from the IRB review requirements [21 CFR 56.105]. Even though FDA has granted a waiver for these drugs, an IRB may still choose to conduct a review under its policies and procedures. The usage of a Group C drug is described in its accompanying "Guideline Protocol" document. The Guideline Protocol contains an FDA-approved informed consent document which must be used if there has been no local IRB review. PARALLEL TRACK The Agency's Parallel Track policy [57 FR 13250] permits wider access to promising new drugs for AIDS/HIV related diseases under a separate "expanded access" protocol that "parallels" the controlled clinical trials that are essential to establish the safety and effectiveness of new drugs. It provides an administrative system that expands the availability of drugs for treating AIDS/HIV. These studies require prospective IRB review and informed consent. EMERGENCY USE IND The need for an investigational drug may arise in an emergency situation that does not allow time for submission of an IND in the usual manner. In such cases, FDA may authorize shipment of the drug for a specified use [21 CFR 312.36]. Such authorization is usually conditioned upon the sponsor filing an appropriate application as soon as practicable. Prospective IRB review is required unless the conditions for exemption are met [21 CFR 56.104(c) and 56.102(d)]. Informed consent is required unless the conditions for exception are met [21 CFR 50.23].
What Causes Chronic Pain? Chronic pain can be caused by many different factors. Often conditions that accompany normal aging may affect bones and joints in ways that cause chronic pain. Other common causes are nerve damage and injuries that fail to heal properly. Some kinds of chronic pain have numerous causes. Back pain, for example, may be caused by a single factor, or any combination of these factors: •Years of poor posture Improper lifting and carrying of heavy objects •Being overweight, which puts excess strain on the back and knees •A congenital condition such as curvature of the spineTraumatic injury •Wearing high heels •Sleeping on a poor mattress •No obvious physical cause •Ordinary aging of the spine (degenerative changes) Disease can also be the underlying cause of chronic pain. Rheumatoid arthritis, osteoarthritis and fibromyalgia are well-known culprits, but persistent pain may also be due to such ailments as cancer, multiple sclerosis, stomach ulcers, AIDS, and gallbladder disease. In many cases, however, the source of chronic pain can be a very complex and even mysterious issue to untangle. Although it may begin with an injury or illness, ongoing pain can develop a psychological dimension after the physical problem has healed. This fact alone makes pinning down a single course of treatment tricky, and it is why health care providers often find they have to try a number of different types of curative steps.

