

Phosphodiesterase 5 Inhibition Attenuates Cerebral Vasospasm and Improves Functional Recovery after Experimental Subarachnoid Hemorrhage
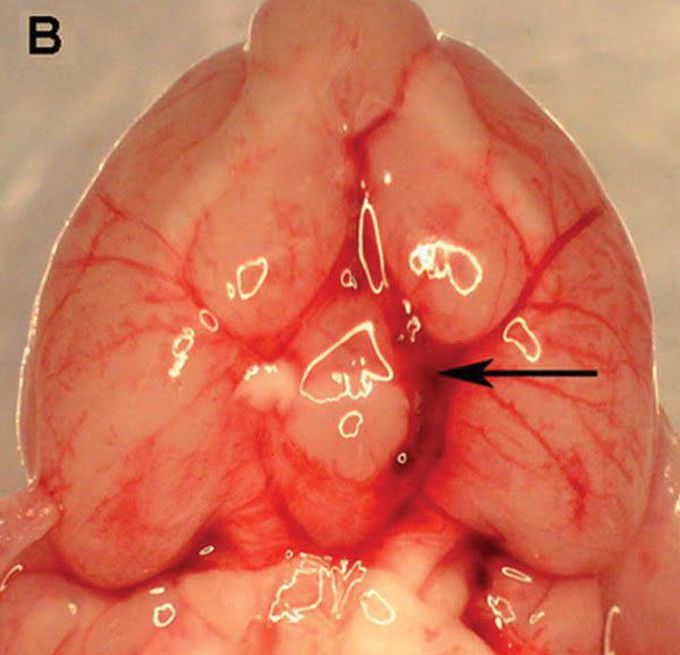
D: Cerebral vasospasm is an independent predictor of poor outcome after subarachnoid hemorrhage (SAH). The nitric oxide-cyclic GMP (NO-cGMP) vasodilatory pathway is strongly implicated in its pathophysiology. Preliminary studies suggest that phosphodiesterase 5 (PDE5) – an enzyme that degrades cGMP – may play a role, as the PDE5 inhibitor sildenafil was found to reduce vasospasm after SAH. However, several questions that are critical when considering translational studies remain unanswered. OBJECTIVE: To elucidate the mechanism of action of sildenafil against vasospasm, and to assess whether sildenafil attenuates SAH-induced neuronal cell death, improves functional outcome after SAH, or causes significant physiological side effects when administered at therapeutically relevant doses. METHODS: SAH was induced via endovascular perforation in male C57BL6 mice. Beginning two hours later, mice received sildenafil citrate (0.7, 2 or 5mg/kg P.O. BID) or vehicle. Neurological outcome was assessed daily. Vasospasm was determined on post-SAH Day 3. Brain PDE5 expression and activity, cGMP content, neuronal cell death, arterial blood pressure (BP), and intracranial pressure (ICP) were examined. RESULTS: We found that PDE5 activity (but not expression) is increased after SAH, leading to decreased cGMP levels. Sildenafil attenuates this increase in PDE5 activity and restores cGMP levels after SAH. Post-SAH initiation of sildenafil was found to reduce vasospasm, decrease neuronal cell death, and markedly improve neurological outcome, without causing significant physiological side effects. CONCLUSION: Sildenafil-an FDA-approved drug with a proven track record of safety in humans -is a promising new therapy for vasospasm and neurological deficits following SAH.

