
Zunaira saleh11 months ago
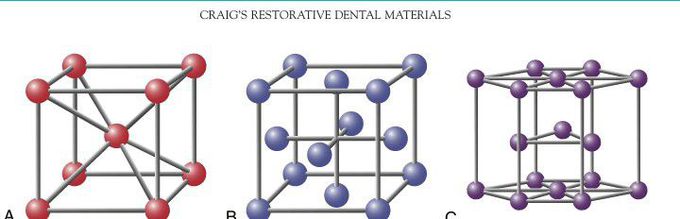
Crystal lattice in dental metals
The three most common crystal lattice unit cells in dental metals and alloys. A, Body-centered cubic cell; B, face-centered cubic cell; and C, hexagonal close-packed cell. The atoms (circles) in all three cases would be larger and touching each other. They were drawn smaller to make the structures easier to visualize.
Other commentsSign in to post comments. You don't have an account? Sign up now!

