
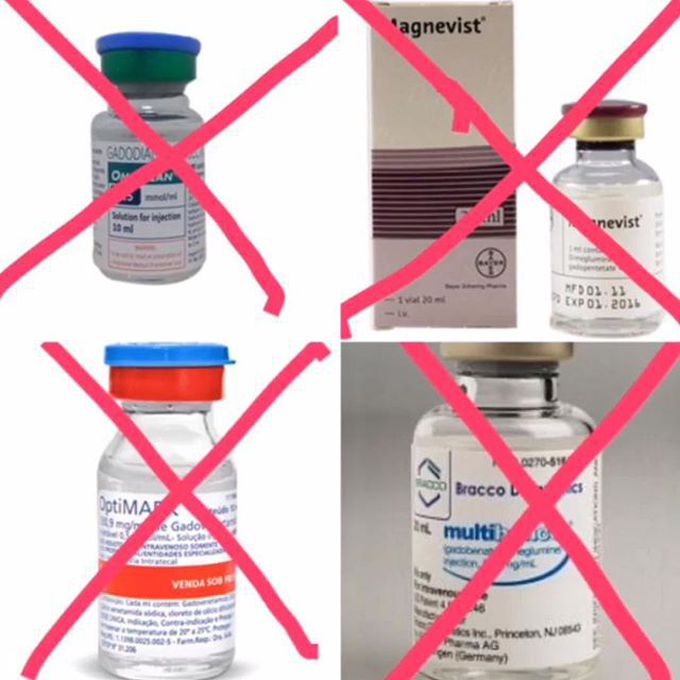
In a stunning development, a European Union regulatory body on March 10 recommended that four gadolinium-based contrast agents (GBCAs) for MRI scans be pulled off the market due to concerns about gadolinium remaining in the body years after scans occur. The agents affected include some of the most widely used contrast products in medical imaging. The four agents covered by the PRAC's (Pharmacovigilance Risk Assessment Committee) ruling this week are all linear gadolinium agents: 1) Gadobenic acid, marketed under the trade name MultiHance by Bracco. 2) Gadodiamide, sold under the trade name Omniscan by GE Healthcare. 3) Gadopentetic acid, marketed as Magnevist by Bayer HealthCare Pharmaceuticals. 4) Gadoversetamide, sold as Optimark by Guerbet. The PRAC made exceptions to its recommendation for two linear gadolinium agents: • Gadoxetate disodium, sold as Primovist in Europe and Eovist in the U.S. by Bayer. The agent is used at a low dose for MRI liver scans and "meets an important diagnostic need in patients with few alternatives." • A formulation of gadopentetic acid (Magnevist) that's injected directly into joints at very low gadolinium concentrations. Full text: http://www.auntminnie.com/index.aspx?sec=sup&sub=mri&pag=dis&ItemID=116837 #MRI #MRI_safety #Gd #gadolinium #GBCAs

